 Review Article
Review Article
Advantageous and Disadvantageous of Non-Classical Biomanipulation
Muhammad Amjad Yaqoob1* , Mingyou Li1 and Syed Shafat Hussain2
1Key Laboratory of integrated Rice-Fish Farming, Ministry of Agriculture and Rural Affairs, Shanghai Ocean University, China
1Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Shanghai Ocean University, China
2Department of Fisheries and Aquaculture, University of veterinary and Animal Sciences, Lahore, Pakistan
Muhammad Amjad Yaqoob, Key Laboratory of integrated Rice-Fish Farming, Ministry of agriculture and Rural Affairs, Shanghai Ocean University, China.
Received Date:January 31, 2023; Published Date:February 13, 2023
Abstract
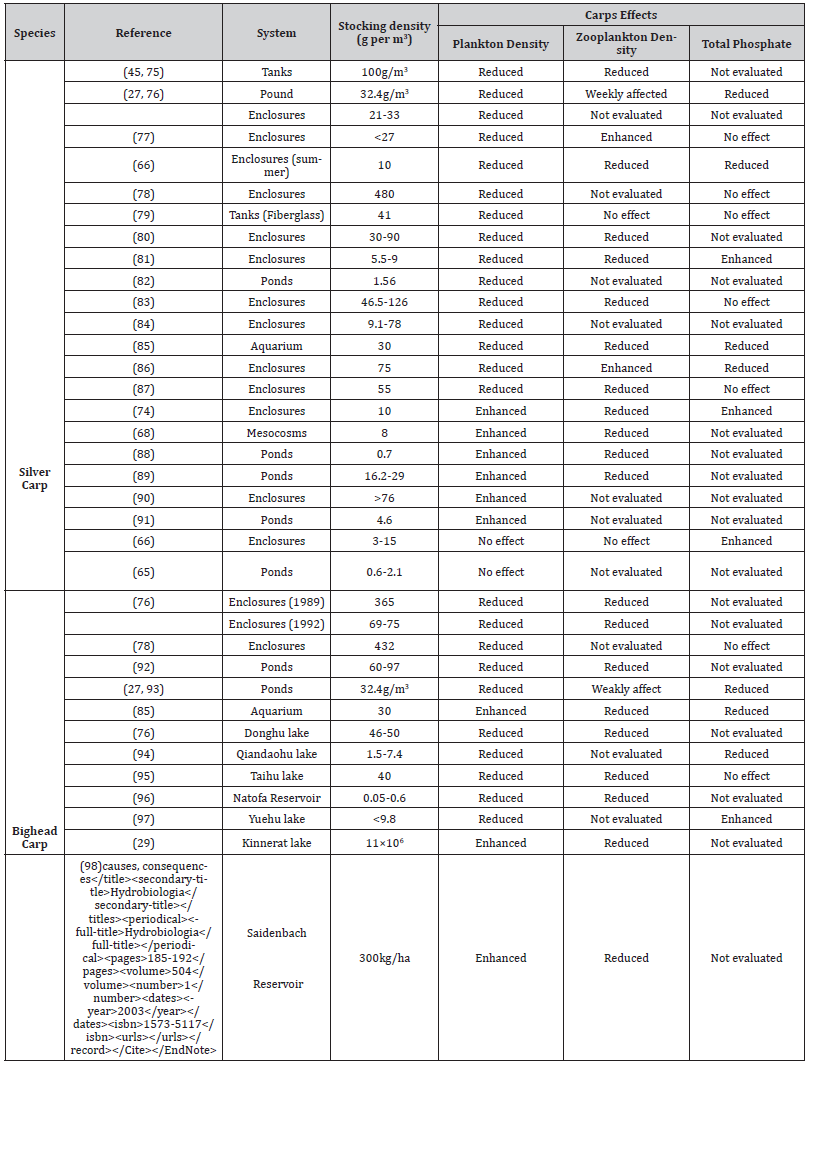
In classical biomanipulation, piscivores fish is introduced to a water body which fed on planktivorous fish and causes an increase in the numbers of zooplankton and zooplankton by feeding on phytoplankton roots to reduction in algal blooms. In contrast, during non-classical biomanipulation piscivores fish is removed from the water body and planktivorous fish is introduced which fed on all kinds of plankton and led to improving the water quality parameters by reducing nuisance algae. Here, we examine the management of water quality parameters through non-classical biomanipulation by identifying problems and extract recommendations. Preference is given to non-classical biomanipulation due to certain hindrances with traditional biomanipulation such as planktivorous fish removal, increase in numbers of macrophyte and decrease in phosphorous (external and internal), respectively. In fact, non-classical biomanipulation can also be used to control algal blooms in tropical, highly productive lakes, where reduction in nutrient concentration is almost impossible. In spite of overlapping prey and predator spaces in closed environments, such as in tanks and ponds, similar results of non-classical biomanipulation were also obtained in lake ecosystems as well. Non-classical biomanipulation changed the community composition of phytoplankton at the start of trial in both enclosed water bodies and lakes. In our review of 30 studies, 63% of studies demonstrated successful control of phytoplankton with non-classical biomanipulation. Microcystis blooms were controlled efficiently by two planktivorous fish, bighead carp (Hypophthalmichthys nobilis) and silver carp (Hypophthalmichthys molitrix), in Lakes Qiandaohu and Donghu, respectively. Eutrophic waters that lack sufficient concentration of macrozooplankton can be effectively managed through planktivorous fishes, such as bighead and silver carp. Non-classical biomanipulation is confirmed as an effective tool for the control of those nuisance algal blooms that cannot be controlled efficiently by large bodied herbivorous zooplankton while ineffective with blooms of nanophytoplankton species.
Keywords: Non-classical biomanipulation, Classical biomanipulation, Bighead Carp, Silver Carp, Algal blooms, Filter feeding fish , Planktivorous.
Efficacy of Control of Blue-Green Blooms through Non-Classical Biomanipulation
Restriction of Classical biomanipulation:
Powerful indirect interactions in ecosystems can easily change the organization of an aquatic ecosystem[1, 2]. Biogeochemical cycles of marine ecosystems depend upon trophic forces [3,4], as demonstrated experimentally by biomanipulation [5]. Classical biomanipulation, used to manage water quality of lakes, involves increasing zooplankton populations, especially Daphnia species that usually feed on phytoplankton [6]. Classical biomanipulation can also be achieved through change in community structure by eliminating planktivorous fish (to increase zooplankton populations) and favoring piscivorous fish. Shift in community structure can be achieved by five different methods that can be used collectively or separately: decrease in water level, fish poisoning, fish winter killing, removal of fish and piscivorous fish stocking [7]. It is evident that zooplankton can control phytoplankton, but results can be variable [7-14], as compared to planktivorous fish [5, 8, 15, 16]. Due to peculiar processes and structures which are highly variable, outcome of biomanipulation is difficult to measure quantitatively. Certain factors should be considered for the successful implementation of classical biomanipulation, such as continued elimination of planktivorous fish and low concentration of phosphorus [17], to decrease phytoplankton amount, and high numbers of macrophytes to maintain water quality [18,19]. Thus, for long term success, such biomanipulation should be restricted to certain types of lakes [20, 21].
Non-Classical Biomanipulation: Use of Planktivorous Fish
Scientists have discovered other methods to control the eutrophication of lakes containing blooms of cyanobacteria as compared to classical manipulation which utilized planktivorous zooplankton. Different studies also determined that large sized zooplankton are more efficient in elimination of algae then smaller [22]. Many studies also found that increase in eutrophication due to cyanobacterial blooms in summer is caused by decrease in numbers of zooplankton especially Daphnia [23, 24]. Zooplankton concentration, composition and growth are also effected by toxic cyanobacteria [25]. In eutrophic lakes where concentration of zooplanktons usually decreases in summer and concentration of algal blooms increase rapidly, we can adopt an alternative approach such as use of planktivorous fish for direct elimination of algal blooms which proved very efficient, respectively [26, 27]. Planktivorous filter feeding fish engulf phytoplankton with large quantities of water and filter them from it in buccal cavity eating more than one prey at a time [27]. Filter feeding silver carp is obligatory planktivorous eating only phytoplankton and small particles [28,29]. Studies showed that filter feeding bighead carp is mainly feed on zooplankton [27,30] but shifted its feeding regime towards phytoplankton during unavailability of zooplankton [27,31].
Use of planktivorous fish as contrast to piscivorous fish for elimination of blue green algae had been done in closed water body and open lake ecosystem as well. Around sixteen studies [31], found the successful elimination of algae blooms with silver carp and some studies observed no or adverse effect [32-34]. Certain studies lead to controversial situation on the use of silver carp for the management of phytoplankton that increase their number instead of ceasing them [35,36]. Gut content analysis of laboratory fed silver carp demonstrated consumption of phytoplankton and zooplankton but not algae [37]. Moreover, sliver carp are behaving like omnivorous instead of planktivorous. While, during high concentration of phytoplankton sliver carp feed like omnivorous and epibenthic browser at low concentration, respectively [38]. However, the change in dynamics and structure of phytoplankton community by filter feeding planktivorous fish through biogeochemical process, predation and zooplankton grazing is well documented. Biogeochemical recycling by filter feeding fish led to two types of factors [39]. Consumption of nutrient by phytoplankton is interacted with planktivorous fish by recycling of nutrient [40]. Indirect prove of nutrient cycling by planktivorous fish was found in different studies [41]. Conversely, nutrient cycling by zooplankton grazing is determined [42]. Literature confirmed that use of planktivorous fish proved successful for the manipulation of phytoplankton. Although, role of zooplankton can also be never separated in lake ecosystem [43].
Phytoplankton Concentration Dynamics Impacted by Nutrient Fluctuation
Algal blooms were observed in St. George Lake (Maine, United States) through nutrient cycling by planktivorous fish [43]. Planktivorous fish did not affect the concentration of zooplankton directly, but rather increased the availability of nutrients by recycling [43,44]. Small sized algae compete with larger sized algae for nutrients, while better surviving the grazing and absorbing more nutrients which are recycled by fish [45]. These factors caused the shift of algae dynamics towards small algae [41]. However, some researchers believed that insufficiency of phosphorous produced from feces of fish in natural ecosystem to alter algae dynamics as high phosphorous rapidly led to bloom [40]. Similarly, recycling of nutrients by zooplankton is another debate. There is also uncertainty in the response of algae to zooplankton and planktivorous fish recycled nutrients [46]. Planktivorous fish led to reduced biomass of zooplankton in contrast to prior studies [43], while nutrients were recycled primarily by zooplankton. Reduction in zooplankton concentration by planktivorous fish is not determined but higher excretion rate was found in small zooplankton as compared to fish [47]. It is believed that nutrient cycling increased indirectly by planktivorous fish by reducing zooplankton biomass. However, phytoplankton production increased exponentially by nutrient recycled by both zooplankton and planktivorous fish respectively [48]. Therefore, different mechanisms such as zooplankton interaction with fish and fish response to phytoplankton would affect the dynamic of phytoplankton [42]. It has been observed that some algae species such as Aphanizomenon and Microcystis which are majorly causes of nuisance blooms get their required phosphorus from gut of planktivorous fish [49]. Further, it was confirmed that Microcystis covered with mucosa remain protected from digestion in gut of planktivorous fish and consume phosphorous required for their growth [50]. While, planktivorous fish as bio cultivar [51] for this bloom forming algal species and increase their survival and photosynthetic ability [51,52]. Incubation of Microcystis into a lake collected from feces of bighead carp, silver carp and tilapia led to eight times increase in colonies cyanobacteria[50]. In contrast to previous studies which were conducted in in-vitro conditions, colonies of Microcystis broken down to single cell in gut of planktivorous fish [53]. In vivo circumstances are totally different and feces containing broken colonies of Microcystis settle at bottom. Several factors are required to release colonies from faces and planktivorous fish has negative effect on algal blooms.
Higher Trophic Level Suppression through Predation
Zooplankton can be suppressed directly by grazing of planktivorous fish and indirectly by decreasing the amount of biomass of algae [45]. Planktivorous fish always led to shift in the community of zooplankton by increasing the density of small zooplankton, such as copepods [54]. The presence of planktivorous fish proved advantageous for the growth of nanophytoplankton and picophytoplankton, as the fish predated on macrozooplankton. This factor ultimately led to an increase in numbers of phytoplankton by suppressing their predators, zooplankton [55]. Alternative situations were observed in eutrophic lakes of subtropical region where silver carp easily controlled the number of phytoplankton and boosted the density of Nano zooplankton [27]. Planktivorous filter feeding fish caused suppressing of zooplankton which is confirmed by numerous studies [26,27,40]. Eight gram per meter cube (8g/m3) is optimum density of silver carp. Below this density, an increase in growth efficiency of phytoplankton causes a harmful effect on the growth of herbivorous zooplankton. Under low water temperatures, the filter feeding efficiency of silver carp remains constant while cyanobacterial blooms are not enough [56]. Reduction in colonial phytoplankton density was observed in the presence of silver carp, despite the presence of crustaceans, due to their selective grazing on small sized phytoplankton [57]. Biomanipulation with silver carp is only efficient at eliminating the algal blooms that cannot be eliminated by herbivorous zooplankton [58]. Isotopic techniques estimate around 45% assimilation rate of Microcystis aeruginosa in silver carp [59]. Similarly, silver carp’s growth and ingestion were noted while fed with toxic species of Microcystis in controlled environment [60]. However, in Taihu Lake fast growth of silver carp was observed with consumption of toxic phytoplankton such as Microcystis up to 84.4% of total phytoplankton consumption [61].
Historical background of non-classical biomanipulation with planktivorous filter feeding fish especially bighead and silver carp regarding water quality management is presented. Through small scale and large-scale experimentation for lake recovery by non-classical biomanipulation was estimated. Due to variation in result of different studies, it is needed to study different lakes with different changing parameters such as composition of food web, trophic condition, geochemistry and development of littoral zone [62]. Spatial and time-based restriction in field experiments led to inappropriate interpretation [63]. These problems can be diminished while studying whole scale community but that is impossible logistically and not quite often. Similarly, same determining factors such as planktivorous fish and nutrients observed in lake experimentation as well [43].
Drawing of theory through small-scale Experiments
Since 1975, almost thirty studies have evaluated the effect of planktivorous fish, such as bighead and silver carp, on the control of phytoplankton. Of those, 63% of studies demonstrated control of phytoplankton with non-classical biomanipulation [27, 37, 54, 57, 62], 9 studies showed no significant change on phytoplankton density [40, 45, 64] and several studies showed no effect on planktivorous [65, 66] (Table 1). The following are the major factors that led to inconsistency in non-classical biomanipulation: temperature [67], stocking density of planktivorous fish [68], composition of phytoplankton community and initial composition of zooplankton [48,67]. Thirty experiments (Table 1) varying in fish stocking density from 0.74 to 480 g/m3 showed no significant relationship of increasing density with reduction of algal blooms. Due to overlapping of planktivorous fish and nutrients, neither of those variables are considered to be a determining factor for the control of phytoplankton [27]. Initial density and composition of phytoplankton and planktivorous fish behavior should be considered for successful management. Diversity in food selection was noted by silver carp [56], which depends solely and strongly on the type of food available in the environment including zooplankton and phytoplankton [69,70]. Silver carp led to reduction in chlorophyll if net phytoplankton dominated the macro phytoplankton density and gain in chlorophyll noted when phytoplankton density is occupied by nanophytoplankton.
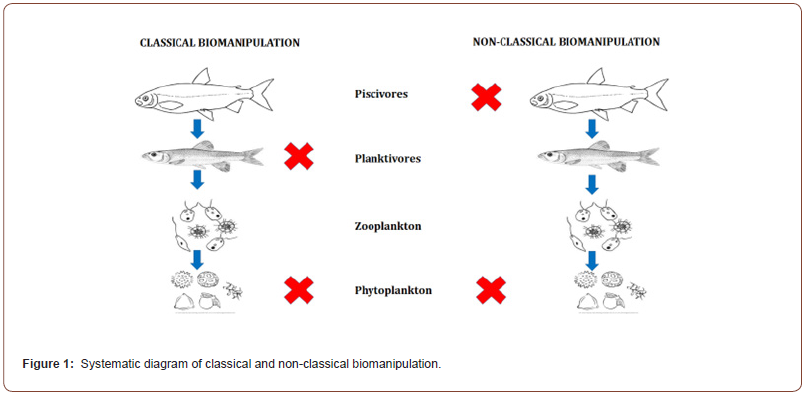
Table 1:Effect of planktivorous fish on zooplankton and phytoplankton density and total phosphate in small scale and large-scale experiments
Classical biomanipulation was more successful in shallow water bodies as compared to deep water bodies [71, 72] while this factor’s impact upon non-classical traditional biomanipulation is not confirmed. However, shallow water bodies lead to overlapping of space which causes increased predator-prey encounters [73]. One non-classical biomanipulation study was conducted in deep water bodies where decreased zooplankton density and increased phytoplankton was noted [74]. Previous studies confirmed that phytoplankton density determined success of non-classical biomanipulation, not the depth of water system [74]. However, for the successful implementation of biomanipulation, phytoplankton composition and density should be noted critically. Non-classical biomanipulation technique can be used for successful maintenance of lakes with high temperature having high density of blue-green algae. Another important concept is that small scale experiments can be helpful in estimation of points, but it should not be applied to large scale experiments [62].
Efficiency of Large-Scale Experiment
Bighead and silver carps are native to eastern Asia and introduced worldwide for culturing purposes and commonly poly-cultured with other fishes [99]. In spite of non-classical biomanipulation for the control of algal blooms, other ecological impacts of bighead and silver carps are not known. Since 1970, bighead and silver carp have been used for control of algal blooms and maintaining water quality in lakes, sewage lagoons and ponds, respectively. Due to the high bone ratio in silver carp and low market demand of these two fish (bighead head and silver carp), people only fish them when other options are not available. Due to the introduction of these fish from Asia, most countries treated them as pest species [65]. Introduced species proved very vulnerable to indigenous species because of no natural predators [100], and introduction proved feasible in community lacking fish species. Invasive species led to changes in ecosystem from mild to very severe viz., hybridization and alteration of habitat and trophic system [101]. In USA, for the protection, welfare and maintenance of indigenous species, the silver and bighead carp are listed in harmful species by Fish and Wildlife Service of the United States, 2007. We describe invasive species as introduced or non-native to ecosystem and cause environmental and economic losses as well as to human health. Just because of high survival and reproductive rate can easily dispersed to wide area. With the establishment of bighead and silver carp in riverine system, it is likely that they can move to lakes as well. Therefore, the stocking of fish should be taken place in proper and effective manner. However, the population of silver and bighead could be kept in check because carps required floating water for the proper development of eggs. If further stocking is stopped, this will lead to gradual decline in fish population respectively. Therefore, silver and bighead carp can be used for maintenance of water quality in eutrophic lakes, respectively.
Inefficient and inappropriate non-classical biomanipulation results of long-term large-scale experiment in lake were determined [29,98] and around five experiments produced efficient and successful results [76, 94-97], as given in (Table 1). Enhancing numbers of indigenous (perch & roach) and exotic (silver carp) planktivorous fish led to decrease in numbers of zooplankton (Daphnia) and increases the density of nanophytoplankton in Ziebach Reservoir of Germany [45]. In lake Pyhaijarvi, change in zooplankton composition led to increase in density of phytoplankton [31,102]. These couple of studies found that a stronger interacting force exists between zooplankton and phytoplankton as compared to phytoplankton and planktivorous fish. Planktivorous fish cause a decrease in density of zooplankton which ultimately boosts the density of small sized phytoplankton which further cause turbidity in water. Similar results were also noted in lake Kinneret of Israel where introduction of fingerlings of silver carp led increase in density of nanophytoplankton by reduction in numbers of zooplankton, respectively [45].
However, successful non-classical manipulation was observed in five different studies in which lakes and reservoirs were bothered by blue green algae and Pyridinium. Cyanobacterial blooms in lake Donghu were successfully eliminated by silver and bighead carp for almost twenty years by gradually increasing the fish density (0.09- 0.11 per m2) to eliminate Microcystis as well as irritating algae, respectively [50]. Nutrients from feces of planktivorous fish are not easily available and optimum for phytoplankton production. So, it proved that nutrients from waste are not important for fish growth [103]. If planktivorous fish feed on zooplankton, then there is a low amount of phosphorous in their bodies and if they fed in littoral zone then high amount of phosphorous available to phytoplankton [104]. The quantitative measurement of nutrient recycling by fish is possible in control or laboratory condition and impossible to measure in natural environment due to dynamic interaction between different food webs.
Experiment in lake Qiandaohu, researcher observed total elimination of algal blooms due to reintroduction of bighead and silver carp. However, decrease in density of planktivorous fish led to cyanobacterial blooms [105]. Similarly, successful biomanipulation with these two carps are also confirmed in lake Taihu at the start of twenty first century [106] and these fish are commonly used in China for biomanipulation, respectively. During summer, a trial was conducted to evaluate the effect of increased stocking density of fish on Microcystis which proved successful. Very slight pathological changes by Microcystis in spite of high blooms were noted in liver of silver carp during study lake Taihu [107]. Fast recovery in liver and kidney tissue of silver confirmed the resistance of silver carp to the toxic cyanobacterial blooms [61,108,109]. That’s why, its universal truth that silver carp can be efficiently used for the elimination of Microcystis blooms and recorded up to 93%, respectively [58]. Change of feeding habitat of organism in lake ecosystem by planktivorous fish make very difficult to made sound prediction of manipulation [110]. Detailed study on food web interaction is required for sound interpretation. Every natural and anthropogenic change in ecosystem has its uniqueness because two lakes can never be same [111]. Because inconsistency in results of large scale experiments regarding water turbidity and fish on phytoplankton, small scale trials are necessary for analyze the management and complexity of food web which set a tool for enhancing water quality through non-classical biomanipulation effectively.
Conclusion
As we know, two different water bodies have their own unique characteristic ecosystem. Although, effectiveness of non-classical manipulation was observed in small scale trials and not as such in large scale. It is because of the complexity of the ecosystem and involvement of different factors such as different interaction in food web. We inference from present review of literature that eutrophic lakes containing blue green algae can easily be maintained by non-classical manipulation except water bodies having nanophytoplankton.
Acknowledgement
The author especially would like to thank the Prof. Dr. Qigen Liu for inspiration, guidance and humble suggestion throughout the journey.
Conflict of Interest
Author declares no conflict of interests.
References
- Chen TC, Ho PC, Gong GC, Tsai AY, Hsieh ChJD (2021) Finding Approaches to Exploring the Environmental Factors That Influence Copepod-Induced Trophic Cascades in the East China Sea. 13: 299.
- Obertegger U, Flaim GJL (2021) A 40-year perspective of an alpine lake: Is everything the same? 91: 125929.
- Chakraborty SK (2021) Trophic Interactions and Biogeochemical Cycles in River Ecosystem. Riverine Ecology 1: 167-234.
- Carpenter SR, Kitchell JF, Hodgson JRJB (1985) Cascading trophic interactions and lake productivity. 35: 634-639.
- Shapiro J. Biomanipulation, an ecosystem approach to lake restoration in (ed). P. 85-96.
- Yin C, He W, Guo L, Gong L, Yang Y, et al. (2022) Can top-down effects of planktivorous fish removal be used to mitigate cyanobacterial blooms in large subtropical highland lakes? 218: 118483.
- Kasprzak P, Gonsiorczyk T, Grossart HP, Hupfer M, Koschel R, et al. (2018) Restoration of a eutrophic hard-water lake by applying an optimised dosage of poly-aluminium chloride (PAC). 70: 33-48.
- Brooks JL, Dodson SI (1965) Predation, Body Size, and Composition of Plankton: The effect of a marine planktivore on lake plankton illustrates theory of size, competition, and predation. science 150: 28-35.
- McQueen DJ, Post JR, Mills EL (1986) Trophic relationships in freshwater pelagic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences. 43: 1571-1581.
- Kerfoot WC, Levitan C, DeMott W (1988) Daphnia‐phytoplankton interactions: density‐dependent shifts in resource quality. Ecology 69: 1806-1825.
- De Bernardi Rd, Giussani G (1990) Are blue-green algae a suitable food for zooplankton? An overview. Hydrobiologia 200: 29-41.
- Lammens EH, Gulati RD, Meijer ML, Van Donk E (1990) The first biomanipulation conference: a synthesis. Biomanipulation Tool for Water Management. 619-628.
- Sarvala J, Ventelae A, Helminen H, Hirvonen A, Saarikari V, et al. (2000) Restoration of the eutrophicated Koeylioenjaervi (SW Finland) through fish removal: whole lake vs. mesocosm experiences. Boreal Environment Research 5: 39-52.
- Taipale SJ, Ventela AM, Litmanen J, Anttila LJE (2022) Poor nutritional quality of primary producers and zooplankton driven by eutrophication is mitigated at upper trophic levels. 12(3): e8687.
- Hambright KD (1994) Morphological constraints in the piscivore‐planktivore interaction: implications for the trophic cascade hypothesis. Limnology and Oceanography 39: 897-912.
- Barth LE, Shuter BJ, Sprules WG, Minns CK, Rusak JAJCJoF (2021) Evaluation of the responsiveness of the crustacean zooplankton community size spectrum to environmental change and an exotic invader in a sample of Canadian Shield lakes. 78: 197-217.
- Zhang Y, Luo P, Zhao S, Kang S, Wang P, et al. (2020) Control and remediation methods for eutrophic lakes in the past 30 years. 81: 1099-1113.
- Goitom E (2018) Ecosystem stability and regime shifts in interconnected shallow lakes.
- Rameshkumar S, Radhakrishnan K, Aanand S, Rajaram RJAWS (2019) Influence of physicochemical water quality on aquatic macrophyte diversity in seasonal wetlands. 9: 1-8.
- Benndorf J, Boing W, Koop J, Neubauer I (2002) Top‐down control of phytoplankton: the role of time scale, lake depth and trophic state. Freshwater biology 47: 2282-2295.
- Bess Z, Chandra S, Suenaga E, Kelson S, Heyvaert AJAS (2021) Zooplankton influences on phytoplankton, water clarity, and nutrients in Lake Tahoe. 83: 1-15.
- Sterner RW (1989) The role of grazers in phytoplankton succession. Plankton ecology pp. 107-170.
- Tanaka Y, Oda S, Nakamura K, Suzuki NJET (2020) A 3‐Species Aquatic Community Model for Ecological Risk Assessment Using Basic Ecotoxicity Data. 39: 1086-1100.
- Li C, Feng W, Chen H, Li X, Song F, et al. (2019) Temporal variation in zooplankton and phytoplankton community species composition and the affecting factors in Lake Taihu—a large freshwater lake in China. 245: 1050-1057.
- Josue II, Cardoso SJ, Miranda M, Mucci M, Ger KA, et al. (2019) Cyanobacteria dominance drives zooplankton functional dispersion. 831: 149-161.
- Shen R, Gu X, Chen H, Mao Z, Zeng Q, et al. (2020) Combining bivalve (Corbicula fluminea) and filter-feeding fish (Aristichthys nobilis) enhances the bioremediation effect of algae: An outdoor mesocosm study. 727: 138692.
- Zhang Z, Shi Y, Zhang J, Liu QJES, Research P (2022) Experimental observation on the effects of bighead carp (Hypophthalmichthys nobilis) on the plankton and water quality in ponds. p. 1-18.
- Lazzaro X (1987) A review of planktivorous fishes: their evolution, feeding behaviours, selectivities, and impacts. Hydrobiologia 146: 97-167.
- Spataru P, Gophen M (1985) Feeding behaviour of silver carp Hypophthalmichthys molitrix Val. and its impact on the food web in Lake Kinneret, Israel. Hydrobiologia 120: 53-61.
- Su C, Hu W, Hu Z, Zhang Z, Wedchaparn O, et al. (2019) Comparison of high-throughput sequencing analysis of gut contents between silver carp Hypophthalmichthys molitrix and bighead carp Hypophthalmichthys nobilis in mesotrophic and eutrophic lakes. 71: 761-770.
- Lin Q, Zeng D, Guo T, Peng LJWR (2021) Filter-feeding fish (Hypophthalmichthys molitrix) mediated phosphorus recycling versus grazing pressure as drivers of the trophic cascade in large enclosures subsidized by allochthonous detritus. 204: 117579.
- Moustaka Gouni M, Sommer UJW (2020) Effects of harmful blooms of large-sized and colonial cyanobacteria on aquatic food webs. 12: 1587.
- Lamptey BL, Sackey AD, Kpabitey KM (2021) Outlining the causes and effects of Algae bloom at Ghana’s West Coast.
- Nair SM, Sajina AJBRT (2021) Fish as Bio-Control Agent for Microalgae and Macrophytes in Various Aquatic Ecosystems. 3: 1133-1136.
- Zubcov E, Andreev N, Ungureanu L, Miron LD, Bagrin N, et al. Maintaining of good water quality–a prerequisite for healthy farmed fish. p. 23-28. In (ed).
- Altenritter ME, DeBoer JA, Maxson KA, Casper AF, Lamer JTJJoem (2022) Ecosystem responses to aquatic invasive species management: A synthesis of two decades of bigheaded carp suppression in a large river. 305: 114354.
- Jawad LA, Faddagh Ziyadi MS, Al Faisal AJ (2021) The Common Carp, Cyprinus carpio: Effect on the Environment and the Indigenous Fish Species in Iraq. Tigris and Euphrates Rivers: Their Environment from Headwaters to Mouth. pp. 877-896.
- Mao Z, Gu X, Cao Y, Luo J, Zeng Q, et al. (2021) Pelagic energy flow supports the food web of a shallow lake following a dramatic regime shift driven by water level changes. 756: 143642.
- Hill Cruz M, Kriest I, Jose YS, Kiko R, Hauss H, et al. (2021) Zooplankton mortality effects on the plankton community of the northern Humboldt Current System: sensitivity of a regional biogeochemical model. 18: 2891-2916.
- Mao Z, Gu X, Cao Y, Zhang M, Zeng Q, et al. (2020) The role of top-down and bottom-up control for phytoplankton in a subtropical shallow eutrophic lake: evidence based on long-term monitoring and modeling. 23: 1449-1463.
- Viitasalo M, Bonsdorff EJESD (2022) Global climate change and the Baltic Sea ecosystem: direct and indirect effects on species, communities and ecosystem functioning. 13: 711-747.
- Ersoy Z, Brucet S, Bartrons M, Mehner TJPo (2019) Short-term fish predation destroys resilience of zooplankton communities and prevents recovery of phytoplankton control by zooplankton grazing. 14: e0212351.
- Dantas DD, Rubim PL, de Oliveira FA, da Costa MR, de Moura CG, et al. (2019) Effects of benthivorous and planktivorous fish on phosphorus cycling, phytoplankton biomass and water transparency of a tropical shallow lake. 829: 31-41.
- Lorenz P, Trommer G, Stibor HJFB (2019) Impacts of increasing nitrogen: phosphorus ratios on zooplankton community composition and whitefish (Coregonus macrophthalmus) growth in a pre‐alpine lake. 64: 1210-1225.
- Shen R, Gu X, Chen H, Mao Z, Zeng Q, et al. (2021) Silver carp (Hypophthalmichthys molitrix) stocking promotes phytoplankton growth by suppression of zooplankton rather than through nutrient recycling: An outdoor mesocosm study. 66: 1074-1088.
- Taipale SJ, Kuoppamaki K, Strandberg U, Peltomaa E, Vuorio KJH (2020) Lake restoration influences nutritional quality of algae and consequently Daphnia biomass. 847: 4539-4557.
- Yu J, Xia M, Zhen W, Shen R, He H, et al. (2020) Density-dependent effects of omnivorous bitterling (Acheilognathus macropterus) on nutrient and plankton communities: implications for lake management and restoration. 847: 3309-3319.
- Boersma M, Meunier CL (2020) Zooplankton-Phytoplankton Interactions in a Changing World. Zooplankton Ecology. CRC Press. P. 28-52.
- Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JM, et al. (2018) Cyanobacterial blooms. 16: 471-483.
- Riba M, Kiss Szikszai A, Gonda S, Boros G, Vital Z, et al. (2019) Microcystis chemotype diversity in the alimentary tract of bigheaded carp. 11: 288.
- Benayache NY, Nguyen Quang T, Hushchyna K, Mc Lellan K, Afri Mehennaoui F Z, et al. SNAoIWE 2019 An overview of cyanobacteria harmful algal bloom (CyanoHAB) issues in freshwater ecosystems 1-25.
- Kolmakov VI, Gladyshev MI 2003 Growth and potential photosynthesis of cyanobacteria are stimulated by viable gut passage in crucian carp. Aquatic Ecology 37: 237-242.
- Pham TL, Utsumi MJJoem 2018 An overview of the accumulation of microcystins in aquatic ecosystems. J Environ Manage 1; 213: 520-529.
- Tillotson NA, Weber MJ, Pierce CLJH 2022 Zooplankton community dynamics along the bigheaded carp invasion front in the Upper Mississippi River. 849: 1659-1675.
- Frau D, Battauz Y, Alvarenga PF, Scarabotti PA, Mayora G, Sinistro RJAE 2019 Assessing the relevance of top-down and bottom-up effects as phytoplankton structure drivers in a subtropical hypereutrophic shallow lake. 53: 265-280.
- Meng LJ, Zhang Y, Li XX, Liu JH, Wen B, et al. 2021 Comparative analysis of bacterial communities of water and intestines of silver carp (Hypophthalmichthys molitrix) and bighead carp (H. nobilis) reared in aquaculture pond systems. 534: 736334.
- Ivan LN, Mason DM, Zhang H, Rutherford ES, Hunter T, et al. 2020 Potential establishment and ecological effects of bighead and silver carp in a productive embayment of the Laurentian Great Lakes. 22:2473-2495.
- Pal M, Yesankar PJ, Dwivedi A, Qureshi AJJoem 2020 Biotic control of harmful algal blooms (HABs): A brief review. J Environ Manage 15; 268: 110687.
- Peng L, Tang Q, Gu J, Lei L, Chen W, et al. 2020 Seasonal variation of microcystins and their accumulation in fish in two large shallow lakes of China. 29(6): 790-800.
- Rahman Z, Haque M, Rahman MJJAMB 2020 Study on the occurrence and abundance of noxious Microcystis spp. in pangasiid catfish (Pangasianodon hypophthalmus) ponds. 9: 1-8.
- Chen J, Xie P, Zhang D, Ke Z, Yang H 2006 In situ studies on the bioaccumulation of microcystins in the phytoplanktivorous silver carp (Hypophthalmichthys molitrix) stocked in Lake Taihu with dense toxic Microcystis blooms. Aquaculture 261: 1026-1038.
- Setlikova I, Maciarzova S, Blaha M, Policar TJFp, biochemistry 2020 Silver carp (Hypophthalmichthys molitrix) can non-mechanically digest cyanobacteria. 46(2) : 771-776.
- Rogers TL, Munch SB, Stewart SD, Palkovacs EP, Giron Nava A, et al. 2020 Trophic control changes with season and nutrient loading in lakes. Ecol lett 23(8): 1287-1297.
- Yu J, Chen J, Deng X, Wu Z, Yu Z, et al. 2019 Trophic patterns of bighead carp and silver carp follow the seasonality of resource availability. 11: 1429.
- Tucker CS 2006 Low‐density silver carp Hypophthalmichthys molitrix (Valenciennes) polyculture does not prevent cyanobacterial off‐flavors in channel catfish Ictalurus punctatus (Rafinesque). Aquaculture Research 37: 209-214.
- Fukushima M, Takamura N, Sun L, Nakagawa M, Matsushige K, et al. 1999 Changes in the plankton community following introduction of filter‐feeding planktivorous fish. Freshwater Biology 42: 719-735.
- Han M, Dong C, Ma S, Feng C, Lei C, et al. 2021 Food Web Responses to a Cyanobacterial Bloom in a Freshwater Eutrophic Lake. 13: 1296.
- Domaizon I, Devaux J 1999 Experimental study of the impacts of silver carp on plankton communities of eutrophic Villerest reservoir (France). Aquatic Ecology 33: 193-204.
- Anderson CA, Anderson RL, Wang J, Gillespie N, Lampo EG, et al. JTJNAJoFM 2021 Juvenile Silver Carp and Bighead Carp as Forage for Predatory Fish in the LaGrange Reach of the Illinois River.
- Sayed RK, Abd El Aziz NA, Ibrahim IA, Mokhtar DMJMR, Technique 2021 Structural, ultrastructural, and functional aspects of the skin of the upper lip of silver carp (Hypophthalmichthys molitrix). 84(8): 1821-1833.
- Juza T, Duras J, Blabolil P, Sajdlova Z, Hess J, et al. 2019 Recovery of the Velky Bolevecky pond (Plzen, Czech Republic) via biomanipulation-key study for management. 136: 167-176.
- Soares LMV, do Carmo Calijuri MJEM, Software 2022 Restoration from eutrophication in interconnected reservoirs: Using a model approach to assess the propagation of water quality improvements downstream along a cascade system. 105308.
- Fall J, Johannesen E, Englund G, Johansen GO, Fiksen OJE 2021 Predator-prey overlap in three dimensions: cod benefits from capelin coming near the seafloor. 44: 802-815.
- Radke RJ, Kahl U 2002 Effects of a filter‐feeding fish [silver carp, Hypophthalmichthys molitrix (Val.)] on phyto‐and zooplankton in a mesotrophic reservoir: results from an enclosure experiment. Freshwater Biology 47: 2337-2344.
- Smith DW 1985 Biological control of excessive phytoplankton growth and the enhancement of aquacultural production. Canadian Journal of Fisheries and Aquatic Sciences 42: 1940-1945.
- Xie P, Liu J 2001 Practical success of biomanipulation using filter-feeding fish to control cyanobacteria blooms: a synthesis of decades of research and application in a subtropical hypereutrophic lake. The Scientific World Journal 8;1: 337-356.
- Wu L, Xie P, Dai M, Wang J 1997 Effects of silver carp density on zooplankton and water quality: implications for eutrophic lakes in China. Journal of freshwater ecology 12: 437-444.
- Datta S, Jana B 1998 Control of bloom in a tropical lake: grazing efficiency of some herbivorous fishes. Journal of Fish Biology 53: 12-24.
- Starling FLdRM 1993 Control of eutrophication by silver carp (Hypophthalmichthys molitrix) in the tropical Paranoa Reservoir (Brasilia, Brazil): a mesocosm experiment. Hydrobiologia 257: 143-152.
- Kajak Z, WA GL 1975 Influence of the planktonivorous fish Hypophthalmichthys molitrix (Val.) on the plankton and benthos of the eutrophic lake.
- Qi L, Deshang L, Bangxi X 1993 Influence of silver carp (Hypophthalmichthys molitrix) on plankton community in reservoir enclosures. Acta Ecologica Sinica (China).
- Ghosh C, Frijns J, Lettinga G 1999 Performance of silver carp (Hypophthalmicthys molitrix) dominated integrated post treatment system for purification of municipal waste water in a temperate climate. Bioresource technology 69: 255-262.
- Lu M, Xie P, Tang H, Shao Z, Xie L 2002 Experimental study of trophic cascade effect of silver carp (Hypophthalmichthys molitrixon) in a subtropical lake, Lake Donghu: on plankton community and underlying mechanisms of changes of crustacean community. Hydrobiologia 487: 19-31.
- Kim BH, Choi MK, Takamura N 2003 Phytoplankton preferences of young silver carp, Hypophthalmichthys molitrix, in hypereutrophic mesocosms during a warm season. Journal of Freshwater Ecology 18: 69-77.
- Cui F, Lin T, Ma F, Zhang L 2004 Experimental studies on biomanipulation of silver carp and bighead carp in water resources management. J Nannjing Univ Sci Technol 28: 668-692.
- Haizhen W, Yongding L, Bangding X, Dunhai L, Dehui C 2004 Ecological meaning and bloom controlling of different density of silver carp followed with Potamogeton crispus in enclosures. Shui Sheng wu Hsueh bao= Acta Hydrobiologica Sinica 28: 141-146.
- Zhang X, Xie P, Hao L, Guo N, Gong Y, et al. 2006 Effects of the phytoplanktivorous silver carp (Hypophthalmichthys molitrixon) on plankton and the hepatotoxic microcystins in an enclosure experiment in a eutrophic lake, Lake Shichahai in Beijing Aquaculture 257: 173-186.
- Lazareva L 1977 Feeding and growth of the bighead, Aristichthys nobilis, in the waters of Dagestan. J Ichthyol 17: 65-75.
- Opuszynski K 1981 Comparison of the usefulness of the silver carp and the bighead carp as additional fish in carp ponds. Aquaculture 25: 223-233.
- Wang J, Xie P, Takamura N, Xie L, Shao Z, et al. 2004 The picophytoplankton in three Chinese lakes of different trophic status and its relationship to fish populations. Journal of Freshwater Ecology 19: 285-293.
- Milstein A, Ahmed A, Masud O, Kadir A, Wahab M 2006 Effects of the filter feeder silver carp and the bottom feeders mrigal and common carp on small indigenous fish species (SIS) and pond ecology. Aquaculture 258: 439-451.
- Opuszynski K, Shireman J 1993 Food habits, feeding behavior and impact of triploid bighead carp, Hypophthalmichthys nobilis, in experimental ponds. Journal of Fish Biology 42: 517-530.
- Burke JS, Bayne DR, Rea H 1986 Impact of silver and bighead carps on plankton communities of channel catfish ponds. Aquaculture 55: 59-68.
- Liu Qg, Chen Mk, Tong Hy, He Gx, Hong Rh, et al. 2004 Study on the possible cause of water blooming and the bloom-prevention technology in Lake Qiandaohu. Agricultural Sciences in China 3: 627-633.
- Ke Z, Xie P, Guo L, Liu Y, Yang H 2007 In situ study on the control of toxic Microcystis blooms using phytoplanktivorous fish in the subtropical Lake Taihu of China: a large fish pen experiment. Aquaculture 265: 127-138.
- Leventer H, Teltsch B 1990 The contribution of silver carp (Hypophthalmichthys molitrix) to the biological control of Ne tofa reservoirs. Hydrobiologia 191: 47-55.
- Lu K, Jin C, Dong S, Gu B, Bowen SH 2006 Feeding and control of blue-green algal blooms by tilapia (Oreochromis niloticus). Hydrobiologia 568: 111-120.
- Horn W 2003 Long-term development of the crustacean plankton in the Saidenbach Reservoir (Germany)–changes, causes, consequences. Hydrobiologia 504: 185-192.
- Mamuru GLS, Qiang J, Xu PJJoFT, org/10.47363/JFTNS/ NSSJDd. 2021 The Economic and Technical Efficiency of Yellow Catfish (Pelteobagrus Fulvidraco) Production in China. 130: 2-6.
- Boets P, Laverty C, Fukuda S, Verreycken H, Green K, et al. 2019 Intra-and intercontinental variation in the functional responses of a high impact alien invasive fish. 21: 1751-1762.
- Pysek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, et al. 2020 Scientists' warning on invasive alien species. 95: 1511-1534.
- Helminen H, Sarvala JJCJoF, Sciences A 1997 Responses of Lake Pyhajarvi (southwestern Finland) to variable recruitment of the major planktivorous fish, vendace (Coregonus albula). 54: 32-40.
- Morales GA, Azcuy RL, Casaretto ME, Marquez L, Hernandez AJ, et al. 2018. Effect of different inorganic phosphorus sources on growth performance, digestibility, retention efficiency and discharge of nutrients in rainbow trout (Oncorhynchus mykiss). 495: 568-574.
- Lepori FJF, Hydrobiologie ALAf 2019 Effects of zooplankton structure and phosphorus concentration on phytoplankton biomass in a freshwater pelagic food chain. 192: 305-317.
- Kibuye FA, Zamyadi A, Wert ECJHA 2021 A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part II-mechanical and biological control methods. Harm ful algae 109: 102119.
- Cai C, He J, Chen W, Zhang J, Wang Q, et al. Fisheries 2019 Biological manipulation of eutrophication in West Yangchen Lake. 4: 190-197.
- Li X, Li J, Meng F, Yao LJE, Safety E 2019 Hepatotoxicity and immunotoxicity of MC-LR on silver carp. Ecotoxicol Environ Saf 169: 28-32.
- Li L, Xie P, Chen J 2007 Biochemical and ultrastructural changes of the liver and kidney of the phytoplanktivorous silver carp feeding naturally on toxic Microcystis blooms in Taihu Lake, China. Toxicon 49(7): 1042-1053.
- Qu X, Hu M, Shang Y, Pan L, Jia P, et al. 2018 Liver transcriptome and miRNA analysis of silver carp (Hypophthalmichthys molitrix) intraperitoneally injected with microcystin-LR. 9: 381.
- Whitfield AK, Able KW, Blaber SJ, Elliott M, Franco A, at al. 2022 Feeding Ecology and Trophic Dynamics. 1: 255-331.
- Huang S, Zhang K, Lin Q, Liu J, Shen JJE-SR 2022 Abrupt ecological shifts of lakes during the Anthropocene. 227: 103981.
-
Muhammad Amjad Yaqoob* , Mingyou Li and Syed Shafat Hussain. Advantageous and Disadvantageous of Non-Classical Page 2 of 9 Biomanipulation. Ad Oceanogr & Marine Biol. 3(3): 2022. AOMB.MS.ID.000561
-
: Non-classical biomanipulation, Classical biomanipulation, Bighead carp, Silver carp, Algal blooms, Filter feeding fish and Planktivorous
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






